Beyond Surface Area – The Architecture of Adsorption
Activated carbon is synonymous with high surface area for engineers and technical professionals in the purification industry. It’s a common and accurate descriptor, but it only tells part of the story. The actual performance of an activated carbon adsorbent is not just a function of how much surface area it has, but how that surface area is architecturally arranged. The internal pore structure—a complex network of voids of varying sizes—is the master blueprint that dictates which contaminants can be captured, how quickly, and how efficiently.
Understanding this internal architecture is the key to moving beyond a generic product selection and toward a precisely engineered purification solution. This guide provides a technical breakdown of the three critical tiers of porosity—micropores, mesopores, and macropores—and explains how their specific functions and distribution determine the ultimate performance of the activated carbon.
Here’s what you will learn:
- The foundational principles of the pore network and its role in adsorption.
- A functional breakdown of micropores, mesopores, and macropores.
- Different raw materials (coal, coconut, wood) naturally produce different pore structures.
- Actionable guidance on matching the proper pore structure to your specific application.
- Advanced concepts like pore volume and pore surface chemistry further refine performance.
1. The Foundation: What Are Pores and Why Do They Matter?
Defining the Pore Network
The defining characteristic of activated carbon is its highly porous nature. The pore network is a vast system of interconnected voids and channels within each carbon granule, created during the high-temperature activation. This process carves out an intricate internal structure, transforming a dense solid into a material mostly space, ready to capture and hold contaminants.
Adsorption vs. Absorption
To understand how pores work, it’s crucial to distinguish between two similar-sounding terms. Absorption is when a substance diffuses into the bulk of a liquid or solid (like a sponge soaking up water). Adsorption, the mechanism by which activated carbon works, is a surface phenomenon where molecules of a substance (the adsorbate) adhere to the surface of another (the adsorbent). The pore network’s function is to maximize the available surface area for this adhesion to occur.
The Surface Area Analogy
The pore network creates an astonishingly large surface area within a minimal volume. A single gram of activated carbon can have an internal surface area of 500 to 1,500 square meters. In perspective, the surface area in just four grams of activated carbon can be equivalent to an entire football field. This massive surface is where the work of purification happens, providing countless sites for contaminant molecules to be trapped and held.
2. The Three Tiers of Porosity: A Functional Breakdown
The International Union of Pure and Applied Chemistry (IUPAC) classifies pores into three main size categories. Each category serves a distinct and vital function within the activated carbon particle, working together in a hierarchical system to efficiently capture contaminants.
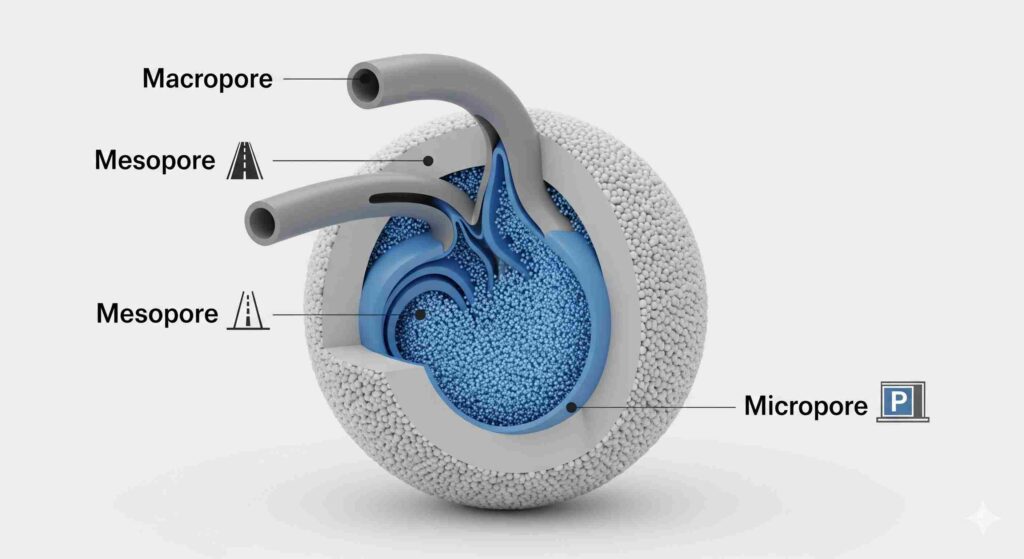
Micropores (<2 nm): The High-Capacity Adsorption Sites
Micropores are the smallest pores and are the true workhorses of adsorption. They constitute most activated carbon’s internal surface area. They are perfectly sized to trap small- to medium-sized molecules through strong attractive forces. Because of their size, they provide the highest adsorption energy, making them exceptionally effective at removing low-concentration organic compounds, volatile organic compounds (VOCs), and disinfection byproducts from liquid and gas streams.
Mesopores (2-50 nm): The Transport Pathways
Mesopores are the intermediate-sized pores that transport the arteries within the carbon particle. They are too large to provide the high-energy adsorption sites found in micropores, but are essential for performance. Their primary role is to serve as conduits, allowing contaminant molecules to travel from the larger macropores on the exterior of the particle deep into the internal structure where the micropores are located. A well-developed mesopore network ensures that the full capacity of the micropores can be utilized efficiently.
Macropores (>50 nm): The Superhighways into the Carbon
Macropores are the most prominent pores and main entry points into the carbon granule. These “superhighways” provide low-resistance pathways for contaminant molecules to quickly diffuse from the bulk fluid into the particle’s interior. They contribute very little to the total surface area. However, they are critical because they prevent the particle’s outer surface from clogging. They are the primary sites for adsorbing huge molecules, such as the color bodies of sugars and chemicals.
| Pore Type | Size Range (IUPAC) | Primary Function | Functional Analogy |
|---|---|---|---|
| Micropores | < 2 nanometers | High-capacity adsorption of small molecules | Parking Spaces |
| Mesopores | 2 – 50 nanometers | Transport of molecules to micropores | City Streets |
| Macropores | > 50 nanometers | Initial entry and adsorption of large molecules | Superhighways |
3. How Raw Materials Engineer the Pore Structure
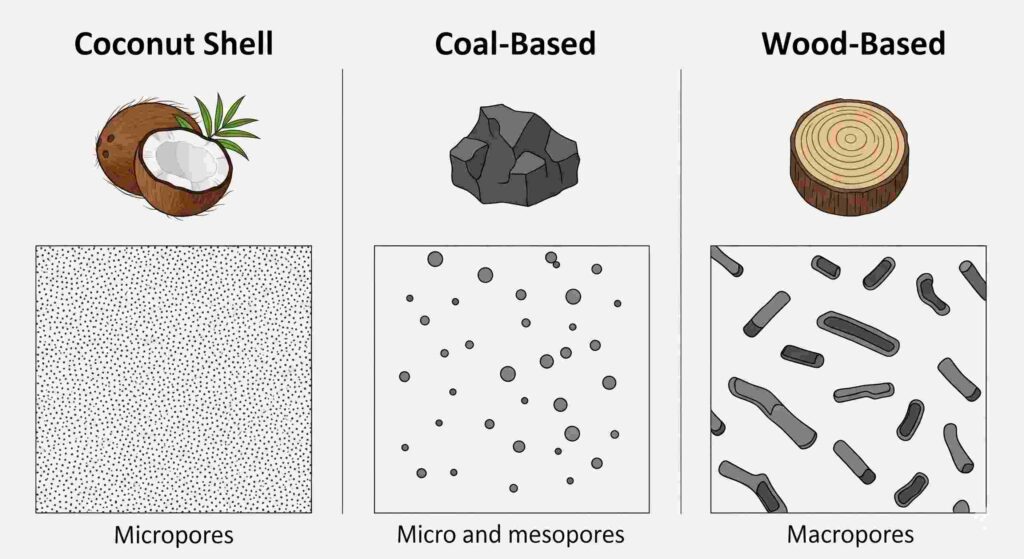
An activated carbon’s final pore size distribution is not accidental; it is primarily determined by the cellular structure of the raw material from which it is made. The three primary sources—coconut shell, coal, and wood—produce a distinct pore architecture.
Coconut Shell-Based Carbon: The Micropore Specialist
Coconut shell is a tough, dense, and highly cellulosic material. The activation process on this dense precursor results in an activated carbon with a predominantly microporous structure.4 This makes coconut shell-based carbon exceptionally effective at removing small organic molecules, such as the trihalomethanes (THMs) in drinking water or low-molecular-weight VOCs in air purification systems.
Coal-Based Carbon: The Versatile All-Rounder
Bituminous and anthracite coal have a more varied and complex starting structure. As a result, coal-based activated carbon features a balanced distribution of micropores and mesopores.4 This versatile pore structure makes it a highly effective “all-rounder,” capable of adsorbing a wide range of molecule sizes. It is the go-to choice for general water treatment, where various organic contaminants of different sizes must be removed.
Wood-Based Carbon: The Macropore Expert
Wood has a much lower density and a more open cellular structure than coal or coconut shell. The activation of this material yields an activated carbon with a well-developed network of macropores and mesopores. While it has fewer micropores, this larger pore structure makes wood-based carbon the ideal choice for liquid-phase decolorization, as it can easily accommodate the large color bodies found in products like sweeteners, juices, and pharmaceuticals.
4. Application-Driven Performance: Matching the Pore to the Pollutant
The most effective way to select an activated carbon is to match its dominant pore structure to the size of the target contaminant molecule. This application-driven approach ensures optimal performance and cost-effectiveness.
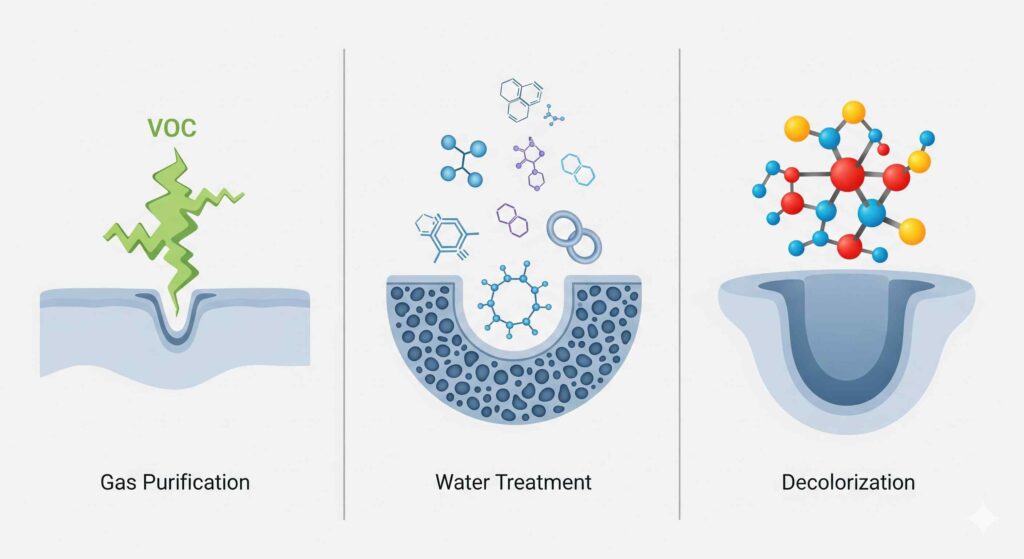
Gas Purification & VOC Removal
Volatile Organic Compounds (VOCs) are typically small, low-molecular-weight molecules. Effective removal requires an adsorbent with a high volume of micropores to trap and hold these molecules securely. Carbons with a predominantly microporous structure, such as those derived from coconut shell, are the superior choice for these applications.
General Water Treatment (Taste, Odor, Organics)
Municipal and industrial water sources often contain a complex mixture of organic contaminants with a wide range of molecular sizes. A versatile carbon with a balanced distribution of micropores (for small taste and odor compounds) and mesopores (for larger natural organic matter) is required. Coal-based activated carbon is the industry standard for this reason.
Liquid Phase Decolorization (Sugars, Chemicals)
The molecules that cause unwanted color in liquids are typically huge and complex. An activated carbon with a highly developed macroporous and mesoporous network is essential to allow these large molecules to enter the particle and be adsorbed. Wood-based activated carbon excels in these applications.
| Application/Problem | Target Contaminant Size | Optimal Pore Structure | Recommended Carbon Type |
|---|---|---|---|
| Gas Purification / VOC Removal | Small | Predominantly Microporous | Coconut Shell-Based |
| General Water Treatment | Small to Medium | Balanced Micro- and Mesoporous | Coal-Based |
| Liquid Phase Decolorization | Large | Predominantly Macro- and Mesoporous | Wood-Based |
5. Beyond Size: The Importance of Pore Volume and Surface Chemistry
While pore size distribution is the primary driver of performance, two other factors provide a more complete picture of an adsorbent’s capabilities.
Pore Volume: The Measure of Total Capacity
If pore size determines what can be adsorbed, then total pore volume determines how much can be adsorbed. Pore volume, typically measured in cm³/g, represents the total space inside the carbon particle. A higher pore volume means the carbon can hold more contaminants before it becomes saturated and requires replacement or regeneration. It is a direct measure of the carbon’s total loading capacity.
Surface Chemistry: Tailoring Adsorption with Functional Groups
The surface of activated carbon is not inert. The activation process can create various oxygen-containing functional groups on the surface. Furthermore, this surface chemistry can be intentionally modified through post-treatments, such as acid washing. These treatments can increase the number of specific functional groups, enhancing the carbon’s affinity for specific polar contaminants, effectively tailoring the adsorbent for a more targeted purification task.
A Blueprint for Selecting the Right Activated Carbon
The performance of activated carbon is a direct result of its internal architecture. A technical professional can make a far more precise and practical selection by moving beyond the general concept of “surface area” and focusing on the specific functions of micropores, mesopores, and macropores. A “pore-structure-first” approach, which matches the adsorbent’s internal blueprint to the size and nature of the target contaminant, is the most reliable strategy for optimizing any purification process.
Key Takeaways
- Micropores are the primary adsorption sites for small molecules, making them ideal for VOC removal and trace organic contaminant control.
- Mesopores are the essential transport pathways that ensure contaminants can reach the micropores efficiently.
- Macropores are the entry highways into the carbon and are critical for removing large molecules like color bodies.
- The choice of raw material—coconut, coal, or wood—is the primary factor determining the final pore size distribution.
- The most effective selection strategy involves matching the carbon’s dominant pore structure to the target pollutant’s molecular size.

